Conditions Commonly Treated
Head Conditions
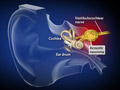
Acoustic Neuroma
Anatomy of the Brain
Arteriovenous Malformation (AVM)
Astrocytoma
Brain Abscess
Brain Aneurysm
Brain Tumor (Overview)
Cerebral Cavernous Malformation (CCM)
Chiari Malformation (CM)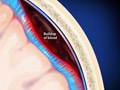
Chronic Subdural Hematoma (Hemorrhage)
Hydrocephalus
Intracerebral Hemorrhage (ICH)
Meningioma
Metastatic Brain Tumor
Myelopathy
Normal Pressure Hydrocephalus (NPH)
Occipital Neuralgia (Arnold's Neuralgia)
Parkinson's Disease (PD)
Pituitary Tumor
Pseudotumor Cerebri
Stroke
Subarachnoid Hemorrhage (SAH)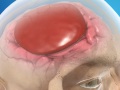
Subdural Hematoma (acute)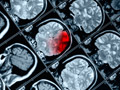
Traumatic Brain Injury (TBI)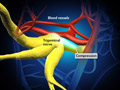
Trigeminal Neuralgia (TN)
Peripheral Conditions
Spine Conditions
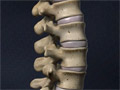
Anatomy of the Spine
Cervical Radiculopathy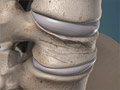
Compression Fractures of the Spine
Degenerative Disc Disease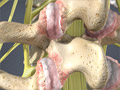
Facet Joint Syndrome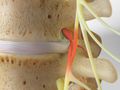
Herniated Disc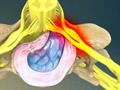
Herniated Disc (Cervical)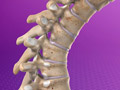
Kyphosis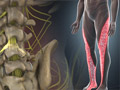
Lumbar Radiculopathy (Sciatica)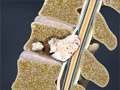
Metastatic Cancer of the Spine
Myelopathy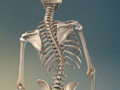
Scoliosis
Spinal Epidural Abscess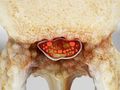
Spinal Stenosis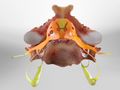
Spinal Stenosis (Cervical)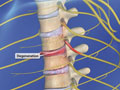
Spinal Stenosis (Thoracic)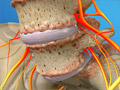
Spondylolisthesis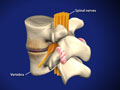
Spondylosis